What are plain language summaries?
Plain language summaries (PLS) in the context of healthcare are concise, easy-to-understand overviews of research findings, written in clear, non-technical language. These summaries translate complex academic concepts into easy-to-understand terminology, making research results more accessible to the general public.
What are the main requirements of a plain language summary?
Typically, there are two main types of PLS within the medical writing industry, collectively referred to as either “PLS” or “PLSP”. We’ve adapted these acronyms to make the differences between each one clear, ensuring the appropriate format is used to effectively communicate research findings to lay audiences:
1. Plain language summary (PLS)
A clear and concise summary of a research study (~500 words) using non-technical language, used for grant applications, research ethics applications, clinical trial registries, or in support of a publication.
2. Plain language summary of publication (PLSP)
A standalone peer-reviewed article summarising a previously published article, using non-technical language and graphic design to enhance visual communication.
3. Plain language summary of a clinical study protocol (PLSS)
A 2-page summary of a clinical study protocol (CSP) that is understandable to a general audience. PLSS are mandated by the EU Clinical Trial Regulation (Annex 1) and can be written as part of the CSP, or a separate document submitted with the CSP.
4. Plain language summary of a clinical study report (PLSC)
A detailed summary of the results of a clinical trial written in plain language. PLSC are a mandatory requirement of the EU Clinical Trial Regulation (Annex V) and should be submitted to the EU database within 1 year from the end of the clinical trial.
How is plain language used in medical communications?
Plain language plays a critical role in bridging the gap between academic work and public understanding, particularly in the medical and scientific fields. Plain language is also a vital consideration with over half of adults in the U.S. having a reading level at or below the 6th grade (i.e., an 11-year-old), and 9 out of 10 adults struggling with adequate health literacy.
The ability to convey information in a way that a general audience can understand is a specialist skill. When written well, plain language enables readers to readily understand and effectively use the information they read.
Our team of highly experienced writers are passionate about language, and we follow plain language principles, including:
- Clarity and simplicity: clear, jargon-free language that can be readily understood by a general audience, avoiding complex technical terms and specialised vocabulary.
- Conciseness and brevity: convey key information in as few words as possible, without sacrificing critical details.
- Patient-centric: craft language with the reader’s level of knowledge and needs in mind, anticipating potential areas of confusion or misunderstanding.
- Scientific accuracy: accurately reflect the aims, methods, and conclusions of the original research study or publication.
- Visual communication: use visual elements to reinforce key points, clarify important concepts, and make the content more approachable and memorable.
By providing a clear, jargon-free summary of the key aims of, or the conclusions from, a research study or scientific publication, plain language enables patients, research participants, and the broader public to comprehend important medical information and engage more actively in decision-making processes. This, in turn, can lead to improved treatment adherence, better clinical outcomes, and enhanced trust between the research community and the public.
What additional document types or materials do you need to help you write in plain language?
As experienced medical writers, we have the necessary knowledge and resources to develop high-quality plain language summaries without requiring any additional materials from you. When developing these summaries, we follow established best practices including plain language, readability, health literacy, and Gestalt design principles. By leveraging these proven techniques, we can create plain language summaries that make complex medical research accessible and engaging for a wide audience.
What is the general timeframe/timeline for writing plain language documents?
The exact timeframe can vary depending on the type of plain language summary you need. A 500-word summary can be completed within a week, whereas a PLSP or PLS-C can take in the region of 3 months to complete, depending on the number of review cycles and response time of individual review teams.
Overview of the plain language process
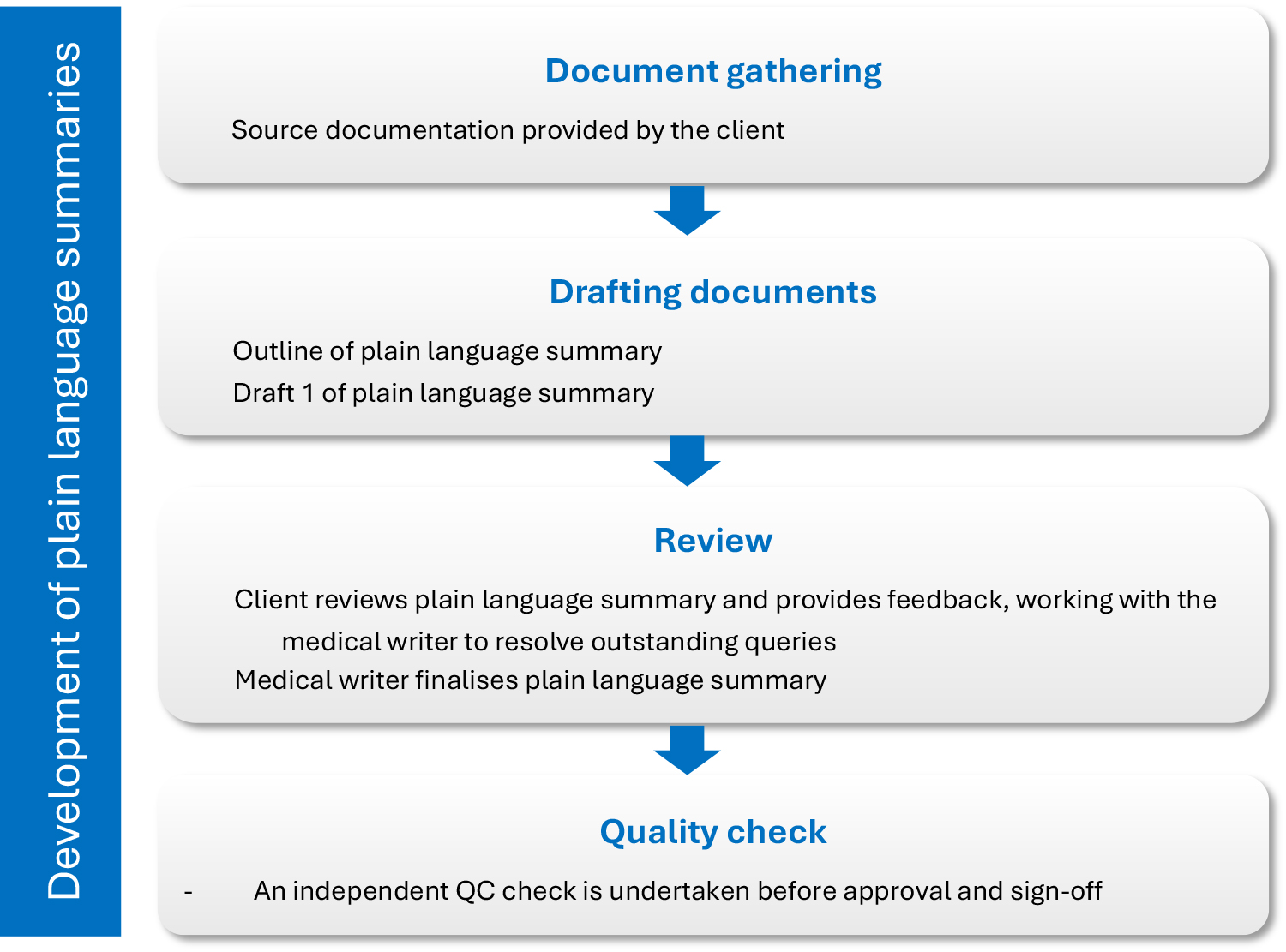
Alchemy’s top tips and tricks for writing in plain language?
Steve Jobs once said: ‘simple can be harder than complex – you have to work hard to get your thinking clean to make it simple.’ That quote describes the essence of plain language in a nutshell. The skill requires extensive reiterative thinking to transform complex data into accessible, patient-centric content – and simplicity is the hallmark of our plain language ability. With almost two decades of combined plain language experience, we can help to distill even the most complex information into language that speaks directly to your target audience. By working hard to get our thinking ‘clean,’ we can facilitate greater public understanding and participation in clinical research.
How can I contact Alchemy about writing a plain language summary?
To discuss your plain language needs, please contact our team at: Contact
Sources:
Shiely F, Daly A. J Clin Epidemiol. 2023;156:105-112.
Plain Language Association International. What is plain language? Available at: https://plainlanguagenetwork.org/plain-language/what-is-plain-language/. Accessed July 2025.
Center for Heath Care Strategies. Health Literacy Fact Sheets. Available at: https://www.chcs.org/resource/health-literacy-fact-sheets/. Accessed July 2025.
Reading.com. The Role of Visual Aids in Reading Development and Comprehension. Available at: https://blog.reading.com/the-role-of-visual-aids-in-reading-development/. Accessed July 2025.
Clinical Trials Regulation, Expert Group on Clinical Trials. Summaries of clinical trial results for laypersons, 2017. Available at: https://health.ec.europa.eu/system/files/2020-02/2017_01_26_summaries_of_ct_results_for_laypersons_0.pdf. Accessed July 2025.
ISO. Plain Language – Part 1: Governing Principles and Guidelines. Available at: https://www.iso.org/obp/ui#iso:std:iso:24495:-1:ed-1:v1:en. Accessed July 2025.